UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of report (Date of earliest event
reported):
(Exact name of registrant as specified in its charter)
|
(State or other jurisdiction of incorporation or organization) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices) (Zip Code)
(
(Registrant’s telephone number, include area code)
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class: | Trading Symbol | Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01 Regulation FD Disclosure.
On May 28, 2025, Y-mAbs Therapeutics, Inc. (the “Company”) is holding a virtual research and development update (the “R&D Update”) regarding progress across its Radiopharmaceutical business unit. A copy of the slide presentation is furnished as Exhibit 99.1 hereto and incorporated herein by reference.
The information in this Item 7.01 and Exhibit 99.1 of this Current Report on Form 8-K is being furnished to the Securities and Exchange Commission and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to liability under that section, nor shall it be deemed incorporated by reference in any filing under the Exchange Act or the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such a filing.
Item 8.01 OTHER EVENTS.
In connection with the R&D Update, on May 28, 2025, the Company issued a press release discussing complete Part A data from its GD2-Self-Assembly DisAssembly (“SADA”) Phase 1 Clinical Trial (Trial 1001) as well as its strategy for development of its SADA program. The full text of the Company’s press release is filed as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
ITEM 9.01. FINANCIAL STATEMENTS AND EXHIBITS.
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Presentation, dated May 28, 2025. | |
| 99.2 | Press Release, dated May 28, 2025, issued by Y-mAbs Therapeutics, Inc. | |
| 104 | Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Y-MABS THERAPEUTICS, INC. | ||
| Date: May 28, 2025 | By: | /s/ Michael Rossi |
| Michael Rossi | ||
| President and Chief Executive Officer | ||
Exhibit 99.1

Radiopharmaceutical R&D Update May 28, 2025

Disclaimer Forward - Looking Statements This presentation contains forward - looking statements within the meaning of the US Private Securities Litigation Reform Act of 1995 . The forward - looking statements involve substantial risks and uncertainties . All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management and expected market growth are forward - looking statements . The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” “goal,” “objective,” “guidance,” “aim,” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words . Such statements include, but are not limited to, statements about pre - clinical and clinical data, regulatory matters, clinical trial timing and plans, the achievement of clinical and commercial milestones, the potential benefits of the Company’s programs and product candidates, and other statements that are not historical facts . Our product candidates and related technologies are novel approaches to cancer treatment that present significant challenges . Actual results may differ materially from those indicated by such forward - looking statements as a result of various factors, including but not limited to : risks associated with our financial condition and need for additional capital ; the risk that actual results of the Company’s business unit realignment will not be as expected ; risks associated with the Company's development work, including any delays or changes to the timing, cost and success of our product development activities and clinical trials including if we encounter difficulties enrolling patients in our clinical trials ; the risks of delays in FDA and/or EU approval of our drug candidates or failure to receive approval ; the risks related to commercializing any approved new pharmaceutical product including the rate and degree of market acceptance of our product candidates ; development of our sales and marketing capabilities and risks associated with failure to obtain sufficient reimbursement for our products ; risks related to our dependence on third parties including for conduct of clinical testing and product manufacture ; our ability to enter into collaboration or other arrangements with partners ; risks associated with protection of our intellectual property rights ; risks associated with macroeconomic conditions, including the conflict between Russia and Ukraine and Israel and Hamas and sanctions related thereto, international trade policies, including tariffs and trade restrictions, inflation, increased interest rates, uncertain global credit and capital markets and disruptions in banking systems ; and other risks and uncertainties affecting the Company including those described in the "Risk Factors" section included in our Annual Report on Form 10 - K for the fiscal year ended December 31 , 2024 and our Quarterly Report on Form 10 - Q for the quarter ended March 31 , 2025 , in addition to other reports the Company files from time to time with the Securities and Exchange Commission . Any forward - looking statements contained in this presentation speak only as of the date hereof, and the Company undertakes no obligation to update any forward - looking statement, whether as a result of new information, future events or otherwise . 2

Today’s Presenters Mike Rossi President and Chief Executive Officer Natalie Tucker SVP, Radiopharmaceutical Business Unit Head Norman LaFrance, MD Chief Medical and Development Officer 3 Additional Team Members Available During Q&A

Y - mAbs is a Commercial Biopharmaceutical Company with Two Distinct Business Units: DANYELZA and Radiopharmaceuticals 4 DANYELZA RADIOPHARMACEUTICALS

Our VISION for GROWTH 5 Deliver on Promise of Radiopharmaceuticals with Minimal O ff - Target E ffects Fully Operational Theranostic Platform Proprietary Radiohaptens Enabling Multiple Isotope Modularity Investment Favors Development, NOT CAPEX Physician Participation Along the Treatment Journey

We are Positioned to Potentially Disrupt the Existing Approach to Radiopharmaceuticals by Addressing Key Obstacles that Limit Commercial Utilization Leverage Existing Infrastructure • Assembly occurs in vivo • Reduced COGS and overhead Patient - Centric Targeting • Modular design enables isotope flexibility • Dosing scalability Enhance Physician Participation • Allows for surround sound participation from Oncologist and RadOnc /NM Improved Patient Safety • Potential for optimal therapeutic dose with minimal toxicity 6

7 Radiopharmaceuticals: Multiple Potential High Value Inflection Points Anticipated Ahead Increasing commitment, capabilities, and leadership in Radiopharmaceuticals 2025 2026 2027 Realignment into two business units: DANYELZA and Radiopharmaceuticals CD38 - SADA FPI in 1H 2025 GD2 - SADA Trial 1001 Part A Data Readout GD2 - Diagnostic IND Submission in 2H 2025 GD2 - Diagnsotic FPI 1H 2026 GD2 - SADA 1001 IND Amendment* 1H 2026 Initiate GD2 - SADA Bridge Study with new Radiohapten in 1H 2026 Trial 1001 Bridge Study Data Readout with new Radiohapten in 2H 2026 Initiate Dose Escalation Study (Trial 1001, Part B) in 1H 2027 Initiation of GD2 - SADA Pediatric Study (Trial 1002) in 1H 2027 GD2 - SADA Pediatric Trial (Trial 1002) Data Readout in 2H 2027 GD2 - SADA Dose Escalation Study (Trial 1001, Part B) Data Readout in 2H 2027 NEW TARGET: IND submission (mCRC) in 1H 2027 NEW TARGET: FPI New Therapeutic (mCRC) 2H 2027 2024 Increased organizational focus on Radiopharmaceuticals New Executive Team appointed with deep Radiopharma expertise * New IND vs Amendment pending FDA feedback; recommendation for new IND may incur delay of 2 - 3 months

8 Today’s Agenda: Three Key Radiopharmaceutical Updates 1. Trial 1001 Part A – Complete 2. Key Learnings from Molecule Optimization Studies 3. Expanded Development Pipeline Y - mAbs Development

9 Recent Insights Will Be Scaled Across the Platform 1 Met primary objective demonstrating GD2 - SADA - 177 Lu - DOTA is safe and well tolerated 2 GD2 - SADA PK was predicted and with close interpatient cohort repeatability 3 Identified opportunities to streamline study designs and improve operations to accelerate future trials 4 Study insights will benefit entire platform and support strategic advancement of high - value targets

10 Today’s Agenda: Three Key Radiopharmaceutical Updates 1. Trial 1001 Part A – Complete 2. Key Learnings from Molecule Optimization Studies 3. Expanded Development Pipeline Y - mAbs Development

GD2 - SADA Trial 1001 Phase 1 Clinical Trial Background 11

12 Cohort 6 Cohort 5 Cohort 4 Cohort 3 Cohort 2 Cohort 1 1 mg/kg 1 mg/kg 3 mg/kg 1 mg/kg 0.3 mg/kg 0.3 mg/kg GD2 - SADA 3 4 5 5 2 5 Interval (days) • Primary : Establish safety of GD2 - SADA • Secondary : Evaluate dosimetry, PK, and immunogenicity profiles of GD2 - SADA - 177 Lu - DOTA • HR - NB (≥16 y.o .) • SCLC (aged ≥18 y.o .) • Sarcoma (aged ≥16 y.o .) • Melanoma (aged ≥18 y.o .) Key Eligibility Criteria • Recurrent or refractory metastatic solid tumors • Measurable/evaluable disease • ECOG 0 or 1 • Adequate liver, renal, and hematological function and no serious intercurrent illness • No prior systemic treatment within 3 wks of 1 st dose Eligible Indications Cohort Design (7 clinical trial sites) GD2 - SADA Phase 1 Trial 1001, Part A: Study Objectives and Design Objectives Trial 1001 Details Part A

13 Part A Treatment Regimen Was Based on Tumors Selected by CT and Included an Imaging Stage Followed by a Treatment Stage Day 1 GD2 - SADA Protein 0.3, 1.0, or 3.0 mg/kg Day 3+ 177 Lu - DOTA 30 mCi Nuclear Imaging Determination of tumor uptake (in 5 prev. selected) Blood was collected at serial timepoints to assess GD2 - SADA and 177 Lu - DOTA PK and GD2 - SADA immunogenicity Varying clearance interval of 2 - 5 days Positive tumor uptake Day 15+ GD2 - SADA (same concentration) and 177 Lu - DOTA (100 or 200 mCi) with same clearance interval Imaging Stage: Tumor Uptake, PK, Dosimetry Assessment Therapeutic Stage: 100 or 200 mCi 177 Lu - DOTA Pre - Imaging Lesion selection via CT scan (up to 5 selected)

Patient Demographics 14

15 A Total of 22 Patients Were Treated with the GD2 - SADA - 177 Lu - DOTA Complex Patients screened 31 Screen failures ( i. e., no measurable disease, lab values out of inclusion and serious intercurrent illness) 8 Withdrawal from study post Grade 3 AE after first GD2 - SADA protein dose 1 No tumor uptake per protocol 13 Patients Included Withdrawal from study (i.e.. disease - related death, moved to different treatment) 2 Safety Analysis Set Patients Excluded Enrollment Flow Chart Enrolled 23 Received GD2 - SADA Protein 23 Received 177 Lu - DOTA (30 mCi) 22 Positive tumor uptake per protocol 9 Received GD2 - SADA and 177 Lu - DOTA (100 or 200 mCi) 7 Patients completed Day 43 blood draw 5 Imaging Set Source Program: t_disp.sas - output: t_disp.rtf - executed: 26FEB2025

16 Patients Were Heavily Pretreated and Similarly Distributed Across Cohorts Program: t_demog.sas - output: t_demog.rtf - executed: 24APR2025 – data cutoff 22APR2025 N: Number of patients, BMI: Body mass index, ECOG: Eastern Cooperative Oncology Group Performance Status Scale, SCLC: Small - Cell Lung Cancer Non - Target lesions defined per RESIST Protocol 23 patients (safety set) Patient Snapshot: • Female: 11 (47.8%) • Male: 12 (52.2%) • Age (yrs): 47.7 (16 – 76) • Weight (kg): 81.5 (46.1 – 128 • BMI: 28.1 (19.6 – 38.9) • ECOG: 0 (43.5%) 1 (56.5%) Metastatic Sites: • Liver • Bone • Lung • Brain # of Tumor Lesions: • Target lesions: Avg. 3.1 (range: 1 – 5) Tumor Lesion Size: • Avg. diameter per lesion: 30.8 mm (range: 10 – 126) Prior Treatments: • Radiotherapy: 78.3% • Surgery: 95.7% • Chemo: 78.3% • ImmunoTx : 52.2% Prior Treatment Lines: • 3.7 (range: 1 – 9)

17 9 Patients in the Imaging Stage Showed Positive Tumor Uptake Per Protocol Design and Were Eligible for Treatment Stage Overview of patients who showed tumor uptake Cohort 6 (3 - day interval) Cohort 6 (3 - day interval) Cohort 6 (3 - day interval) Cohorts 5 (4 - day interval) Cohort 4 (5 - day interval) Cohort 3 (5 - day interval) Cohort 3 (5 - day interval) Cohort 3 (5 - day interval) Cohort 2 (2 - day interval) Melanoma Uveal Melanoma Melanoma Uveal Melanoma Leyomyosarco ma Uveal Melanoma Synovial Sarcoma Osteosarcoma Osteosarcoma Diagnosis 1.0 1.0 1.0 1.0 3.0 1.0 1.0 1.0 0.3 Dose level (mg/kg) Yes Yes Yes Yes Yes Yes Yes Yes Yes Tumor uptake Tumor Uptake by Tumor Type (N = 22) 4/11 (2/3) Sarcoma All (Osteosarcoma) 5/8 Melanoma 0/1 Small Cell Lung Cancer (SCLC) 0/2 Neuroblastoma (NB)* Data cut as of January 6, 2025. These early results are not complete and are not necessarily indicative of the full results o r u ltimate success of the SADA trials or the SADA development program which is in early development with no guaranty of approval. * Neuroblastoma patients were >16 years old, per protocol with prior GD2 treatments

Safety Summary 18

19 Safety Summary : Part A was Safe and Well - Tolerated Across Both GD2 - SADA and 177 Lu - DOTA Administrations • No AE trends across all dosing cohorts • No DLTs or treatment - related serious adverse events • Treatment related adverse events were mostly CTCAE grade 1 (70%) and 2 (27.5%) • ADA did not show conclusive evidence of immunogenicity safety risks • Most adverse events were lymphocyte count decrease, nausea, and constipation • Most related adverse events were nausea and chills • No dose - dependent trends related to GD2 - or radiation - related adverse events • Two patients reported a total of 3 AEs of Special Interest (AESI) • One non - serious related event (pain) • Two non - related events attributed to disease progression (liver enzymes) 1 2 3 AE: A dverse event , TEAE: Treatment - emergent adverse event , CTCAE: Common terminology criteria for adverse events SAE: Serious adverse event, AESI: Adverse event of special interest, DLT: Dose - limiting toxicity, ADA: Anti - drug antibodies Related AEs are either 'Possibly' or 'Probably' related to trial drug AE DLT SAE TEAE ADA AESI

20 Manageable Safety Profile: No DLTs, No Treatment - related SAEs 0 3 8 16 123 128 0 1 0 1 40 40 0 20 40 60 80 100 120 140 DLT AESI SAE Grade 3+ Any TEAE Any AE Number of Events Adverse Event Type Related AE All AE TEAE: Treatment - emergent adverse event Grade 3+: Common terminology criteria for adverse events (CTCAE) Grade 3 or higher SAE: Serious adverse event AESI: Adverse event of special interest DLT: Dose - limiting toxicity Related AEs are either 'Possibly' or 'Probably' related to trial drug Program: t_ae.sas - output: t_ae.rtf - executed: 24APR2025 - data cutoff: 22APR2025

21 Nausea, Chills Were the Most Common Related Adverse Events 0 1 2 3 4 5 6 7 8 9 10 Nausea Lymphocyte count decreased Constipation Abdominal pain Arthralgia Back pain Chills Hypoalbuminaemia Hyponatraemia Pain in extremity Decreased appetite Fatigue TEAEs with a total frequency ≥ 10% N E 2 2 2 2 2 2 2 3 4 Pain in extremity Lymphocyte count decreased Hypophosphataemia Hot flush Headache Fatigue Arthralgia Chills Nausea Related AEs observed in ≥2 Patients N Grades 1 and 2 Grade 1 Grade 1 Grade 1 Grade 1 Grade 1 Grades 1and 2 Grade 2 Grades 1 and 2 Program: t_ae_soc.sas - output: t_ae_soc_teae.rtf - executed: 24APR2025 - data cutoff: 22APR2025 N: Number of patients experiencing the event at least once, E: Total number of reports of the event PT: Preferred term, TEAE: Treatment - emergent adverse event Note: Related AEs are either 'Possibly' or 'Probably' related to trial drug

22 No Dose - Dependent AE Trends as Seen With GD2 Therapies or Radiopharmaceuticals; AESI Were Non - Serious Patient 1, AESI 1: Abdominal Pain 3 Adverse Events of Special Interest (AESI) in 2 Patients Patient 2, AESIs 2 & 3: Liver Function 5: 1 mg/kg, 4 - day interval Cohort # Hx of elevated liver enzymes History Elevated liver enzymes at enrollment and 1 st infusion reaching grade 3 at end of treatment visit Presentation ALT and AST values remained high despite end of treatment Outcomes Both AESIs were serious and not recovered but not related to study drug and related to disease progression Conclusion 4: 3 mg/kg, 5 - day interval Cohort # History of cancer pain prior to GD2 - SADA treatment and received concomitant medication to manage pain; also hx of diarrhea and nausea History Grade 3 abdominal pain day of 1 st infusion Presentation • Pain was non - serious and the patient recovered on the same day • Patient withdrew from study and did not receive 177 Lu - DOTA during the Imaging Stage Outcomes With only one occurrence of related abdominal pain and no rechallenge, more evidence would be needed to draw any definitive safety conclusions Conclusion AESI: Adverse event of special interest predefined per protocol AST: Aspartate Transaminase [normal range 5 – 34U/L] ALT: Alanine Transaminase [normal range 0 – 55U/L] Bilirubin [normal range 0 – 1.4mg/dL] Related AEs are either 'Possibly' or 'Probably' related to trial drug

GD2 - SADA Pharmacokinetics (PK) 23

24 Pharmacokinetics of GD2 - SADA Outline the Optimal Clearance Interval for Maximum Tumor - to - Kidney Ratio • Allometric scaling was used to model human GD2 - SADA PK based on preclinical data • GD2 - SADA blood trough was identified preclinically , i.e., lowest amount of GD2 - SADA in blood prior to 177 Lu - DOTA (≤1 ug/mL) • Key Learning for Trial: Trough drives toxicity for SADA platform; similar to historic measurement of aminoglycoside trough to avoid renal toxicity Source: “Preclinical and Translational Pharmacokinetics of GD2 - SADA, a Self - Assembling and Disassembling (SADA) Bispecific Fusion Protein for Pretargeted Radioimmunotherapy (PRIT)”, B.H. Santich et. al., SNMMI, Nov. 2024 Preclinical Model Key Takeaways

25 GD2 - SADA Pharmacokinetics are Dose Dependent and Predictably Follow Modeling Source: Trial: Y - mAbs 1001 DMC. 06May2025 Note: Serum levels of GD2 - SADA were measured over time N=22: One patient who withdrew prior to 177 Lu - DOTA administration did not continue with PK sampling • The initial concentration of administered GD2 - SADA Protein correlated with the amount of GD2 - SADA in serum at the Cmax and over time (AUC) • PK highly reproducible when looked at on a per patient basis by cohort Illustrative 1001 GD2 - SADA PK in Serum (n=2 2 ) SADA PK Key Takeaways GD2 - SADA Concentration (ug/mL) Pharmacokinetic Profiles (Safety Analysis Set) GD2 - SADA Concentration (ug/mL) Time Time Since First Dose of GD2 - SADA (hours) Trial: Y-mAbs 1001 DMC. Program: f_pk_sada2.sas - output: f_pk_sada2.rtf - executed: 06MAY2025 0 h o u r s 2 4 h o u r s 4 8 h o u r s 7 2 h o u r s 9 6 h o u r s 1 2 0 h o u r s 1 6 8 h o u r s 1 9 2 h o u r s 2 1 6 h o u r s Time since first dose of GD2-SADA 0 10 20 30 40 50 60 70 80 90 100 G D 2 - S A D A C o n c e n t r a t i o n ( u g / m L ) Cohort 6Cohort 5Cohort 4Cohort 3Cohort 2Cohort 1Cohort: 100 90 80 70 60 50 40 30 20 10 0 0 24 48 72 96 120 168 192 216

26 Dose Normalized GD2 - SADA PK Displayed Proportional Cmax and Clearance Rates over Three Administered Dose Concentrations Source: Trial: Y - mAbs 1001. f_median_sada.rtf - 05May2025 Note: Serum levels of GD2 - SADA were measured over time, normalized by mg/kg of GD2 - SADA protein Time (hours) GD2 - SADA PK – Dose Normalized • When normalized by dose, all protein concentrations tested showed similar clearance rates over time • GD2 - SADA PK provides a predictable roadmap for tailoring clearance interval prior to isotope administration to maximize therapeutic index Key Takeaways Total GD2 - SADA (ug/mL)/(mg/kg) Illustrative SADA PK GD2 - SADA Concentration (ug/mL) Time 0.1 0.3 1 3 10 30 T o t a l G D 2 - S A D A ( u g / m L ) / ( m g / k g ) 24 48 72 96 120 Nominal Time (Hours) 310.3Dose (mg/kg): The error bars represent the geometric standard deviation Trial: Y-mAbs 1001 Program: f_pk4.sas - output: f_gm_sada.rtf - executed: 13MAY2025 0.1 0.3 1 3 10 30 24 48 72 96 120 Normal Time (hours) 0.1 0.3 1 3 10 30 T o t a l G D 2 - S A D A ( u g / m L ) / ( m g / k g ) 24 48 72 96 120 Nominal Time (Hours) 310.3Dose (mg/kg): The error bars represent the geometric standard deviation Trial: Y-mAbs 1001 Program: f_pk4.sas - output: f_gm_sada.rtf - executed: 13MAY2025

177 Lu - DOTA Pharmacokinetics (PK) 27

177 Lu - DOTA PK is a Function of the GD2 - SADA Protein Concentration and Clearance Interval Allowing the Optimization of Therapeutic Index 28 Note: Pre - dose GD2 - SADA concentration is assigned a value of 10, as it is 0 ng/mL for all included records • Higher concentrations of GD2 - SADA in serum correlate with higher radioactivity levels in serum • This effect can be leveraged and applied by extending intervals • Understanding of PK informs clearance interval to optimize therapeutic index Illustrative Hours Since First Dose of GD2 - SADA Log Concentration (ng/mL) GD2 - SADA Concentration followed by 177 Lu - DOTA Concentration by Cohort and Time (Safety Analysis Set) Tumor Site GD2 - SADA PK 177 Lu - DOTA PK 177 Lu - DOTA Key Takeaways GD2 - SADA PK and 177 Lu - DOTA PK

177 Lu - DOTA PK by Cohort Illustrates Dual Impact of GD2 - SADA Concentration and Clearance Interval 29 Note: Pre - dose GD2 - SADA concentration is assigned a value of 10, as it is 0 ng/mL for all included records 177 Lu - DOTA PK by Cohort (safety analysis set) 177 Lu - DOTA • Multiple protein doses over the same interval show GD2 - SADA higher protein concentration correlates with slower 177 Lu - DOTA clearance • Correlative results suggest effective binding of 177 Lu - DOTA to GD2 - SADA in vivo Illustrative Key Takeaways Tumor Site Log Geometric Mean Concentration (ng/mL) Hours After Start of 177 Lu - DOTA Administration Cohort 1 0.3 mg/kg, 5 Days Cohort 3 1.0 mg/kg, 5 Days Cohort 2 – 0.3 mg/kg, 2 days Cohort 6 – 1.0 mg/kg, 3 days Cohort 5 - 1.0 mg/kg, 4 Days Cohort 4 3.0 mg/kg, 5 Days

GD2 - SADA - 177 Lu - DOTA Dosimetry 30

31 Per Protocol, Tumor Uptake Was Restricted to Site Selected Target Lesions Identified via CT; Expanded Evaluation Included All Tumors OLINDA/EXM 2.2 (Organ Level INternal Dose Assessment/ EXponential Modeling), Hermes Medical Solutions Expanded Evaluation Per Protocol Evaluation Next generation imaging provides more insight on heterogenous tumors x Assessment of up to 5 target lesions determined by CT within 21 days prior to first GD2 - SADA dose (measurable per RECIST 1.1) x Tumor uptake assessment conducted locally 24 hours post 177 Lu - DOTA based on qualitative impression of contrast - to - noise ratio >3 x Only target lesions deemed positive for uptake by the site were evaluated for dosimetry x Identify all tumors (target and non - target), leveraging data from SPECT/CT x Conduct organ dosimetry and tumor dosimetry on all tumors OLINDA/EXM® (dose - factor based, v1 FDA clearance 2004) Torch ® advanced dosimetry - guided radiopharmaceutical therapy assessment software (GPU - accelerated, Full Monte Carlo dose analysis, FDA 510(k) cleared 2021)

32 Per Protocol Evaluation: 9 of 22 Patients Were Identified as Having Tumor Uptake Patient 021 Patient 019 Patient 018 Patient 016 Patient 011 Patient 009 Patient 008 Patient 005 Patient 003 6 6 6 5 4 3 3 3 2 Cohort 1.0 1.0 1.0 1.0 3.0 1.0 1.0 1.0 0.3 GD2 - SADA protein dose (mg/kg) 3 3 3 4 5 5 5 5 2 Clearance Interval (days) Melanoma Uveal Melanoma Melanoma Uveal Melanoma Leyomyo - sarcoma Uveal Melanoma Synovial Sarcoma Osteosarcoma Osteosarcoma Diagnosis NA* 0.32 Pending Analysis 0.19 0.07 - 0.12 0.07 0.10 0.03 - 0.05 0.27 - 0.39 Tumor (Gy) SPECT/CT 0.81 1.83 0.38 0.30 2.33 0.14 0.32 0.23 0.70 Kidney (Gy) 0.20 0.68 0.30 0.24 0.08 0.12 0.25 0.01 0.28 Spleen 0.02 0.07 0.01 0.03 0.02 0.01 0.03 0.01 0.04 Red Marrow (Gy) Note: All data based on 30mCi 177 LuDOTA diagnostic dose; Gy represents absorbed dose Patient 21 (NA): Patient had tumor uptake, but lesions too close to heart for dosimetry analysis Patient 18 (pending analysis): data evaluation on hold, pending receipt of target lesion documentation Analysis completed with OLINDA/EXM 2.2 Software

33 Note: All data based on 30mCi 177 LuDOTA diagnostic dose; Gy represents absorbed dose; column colors represents cohorts Patient 21 (pending analysis ): positive tumor uptake confirmed, dosimetry calculations on hold pending receipt of additional imaging data (CT scan ) Patient 22 (pending analysis): organ analysis pending receipt of additional site images (planar/SPECT) Per Expanded Evaluation : 16 of 22 Patients Showed Tumor Uptake, Largely Presenting with Increased Gray per Tumor Patient 022 Patient 017 Patient 015 Patient 014 Patient 013 Patient 006 Patient 004 Patient 021 Patient 019 Patient 018 Patient 016 Patient 011 Patient 009 Patient 008 Patient 005 Patient 003 6 5 5 4 4 3 2 6 6 6 5 4 3 3 3 2 Cohort 1.0 1.0 1.0 3.0 3.0 1.0 0.3 1.0 1.0 1.0 1.0 3.0 1.0 1.0 1.0 0.3 GD2 - SADA protein dose (mg/kg) 3 4 4 5 5 5 2 3 3 3 4 5 5 5 5 2 Clearance Interval (days) Osteo - sarcoma Uveal Melanoma Neuro - sarcoma Ewing Sarcoma Cutaneous Melanoma Pleomorphic Liposarcoma Small Cell Lung Cancer Cutaneous Melanoma Uveal Melanoma Melanoma Uveal Melanoma Leyomyo - sarcoma Uveal Melanoma Synovial Sarcoma Osteo - sarcoma Osteo - sarcoma Diagnosis 0.10 - 1.0 0.20 - 0.40 0.05 - 0.10 0.10 - 0.20 0.08 - 0.10 0.001 - 0.011 0.20 Pending Analysis 0.10 - 0.80 0.20 0.04 - 0.30 0.08 - 0.20 0.30 0.30 0.06 - 0.30 0.40 - 1.10 Tumor (Gy) SPECT/CT Pending Analysis 0.20/0.20 0.30/0.30 0.30/0.40 0.6/0.50 0.01/0.01 0.80/0.80 Pending Analysis 1.70/1.80 0.20/0.20 0.2/0.2 0.90/0.80 0.30/0.40 0.30/0.30 0.30/0.10 1.0/1.5 Kidney (Gy) Pending Analysis 0.20 0.10 0.30 0.20 0.01 0.40 Pending Analysis 0.90 0.10 0.10 0.30 0.20 0.20 0.10 0.50 Spleen Pending Analysis 0.20 0.10 0.20 0.10 0.002 0.20 Pending Analysis 0.03 0.04 0.08 0.01 0.10 0.10 0.07 0.20 Lumbar Marrow (Gy) Analysis completed with Torch® Software Per protocol analysis set Expanded analysis set

34 Per Expanded Evaluation : 16 of 22 Patients Showed Tumor Uptake, Largely Presenting with Increased Gray per Tumor Patient 022 Patient 017 Patient 015 Patient 014 Patient 013 Patient 006 Patient 004 Patient 021 Patient 019 Patient 018 Patient 016 Patient 011 Patient 009 Patient 008 Patient 005 Patient 003 6 5 5 4 4 3 2 6 6 6 5 4 3 3 3 2 Cohort 1.0 1.0 1.0 3.0 3.0 1.0 0.3 1.0 1.0 1.0 1.0 3.0 1.0 1.0 1.0 0.3 GD2 - SADA protein dose (mg/kg) 3 4 4 5 5 5 2 3 3 3 4 5 5 5 5 2 Clearance Interval (days) Osteo - sarcoma Uveal Melanoma Neuro - sarcoma Ewing Sarcoma Cutaneous Melanoma Pleomorphic Liposarcoma Small Cell Lung Cancer Cutaneous Melanoma Uveal Melanoma Melanoma Uveal Melanoma Leyomyo - sarcoma Uveal Melanoma Synovial Sarcoma Osteo - sarcoma Osteo - sarcoma Diagnosis 0.10 - 1.0 0.20 - 0.40 0.05 - 0.10 0.10 - 0.20 0.08 - 0.10 0.001 - 0.011 0.20 Pending Analysis 0.10 - 0.80 0.20 0.04 - 0.30 0.08 - 0.20 0.30 0.30 0.06 - 0.30 0.40 - 1.10 Tumor (Gy) SPECT/CT Pending Analysis 0.20/0.20 0.30/0.30 0.30/0.40 0.6/0.50 0.01/0.01 0.80/0.80 Pending Analysis 1.70/1.80 0.20/0.20 0.2/0.2 0.90/0.80 0.30/0.40 0.30/0.30 0.30/0.10 1.0/1.5 Kidney (Gy) Pending Analysis 0.20 0.10 0.30 0.20 0.01 0.40 Pending Analysis 0.90 0.10 0.10 0.30 0.20 0.20 0.10 0.50 Spleen Pending Analysis 0.20 0.10 0.20 0.10 0.002 0.20 Pending Analysis 0.03 0.04 0.08 0.01 0.10 0.10 0.07 0.20 Lumbar Marrow (Gy) Tumor Uptake by Tumor Type (N = 22) 8/11 (3/3) Sarcoma All (Osteosarcoma) 7/8 Melanoma 1/1 Small Cell Lung Cancer (SCLC) 0/2 Neuroblastoma (NB) Per protocol analysis set Expanded analysis set Analysis completed with Torch® Software Note: All data based on 30mCi 177 LuDOTA diagnostic dose; Gy represents absorbed dose; column colors represents cohorts Patient 21 (pending analysis ): positive tumor uptake confirmed, dosimetry calculations on hold pending receipt of additional imaging data (CT scan ) Patient 22 (pending analysis): organ analysis pending receipt of additional site images (planar/SPECT)

35 Protocol Artificially Restrained Tumor Selection and Resulted in Additional Tumors with Dose Uptake Excluded from Evaluation Positive Patient: Nontarget Lesion with Uptake Patient ### - ## - #### - 011: Cohort 5, 1 mg/kg GD2 - SADA + 3 - day clearance interval, Uveal Melanoma Patient continued onto Therapy stage as other target lesions showed uptake Negative Patient: Nontarget Lesion with Uptake Patient 100 - 48 - 1001 - 001: Cohort 4, 3 mg/kg GD2 - SADA + 5 - day clearance interval, Cutaneous Melanoma Patient stopped at Imaging stage because uptake was on non - target lesions
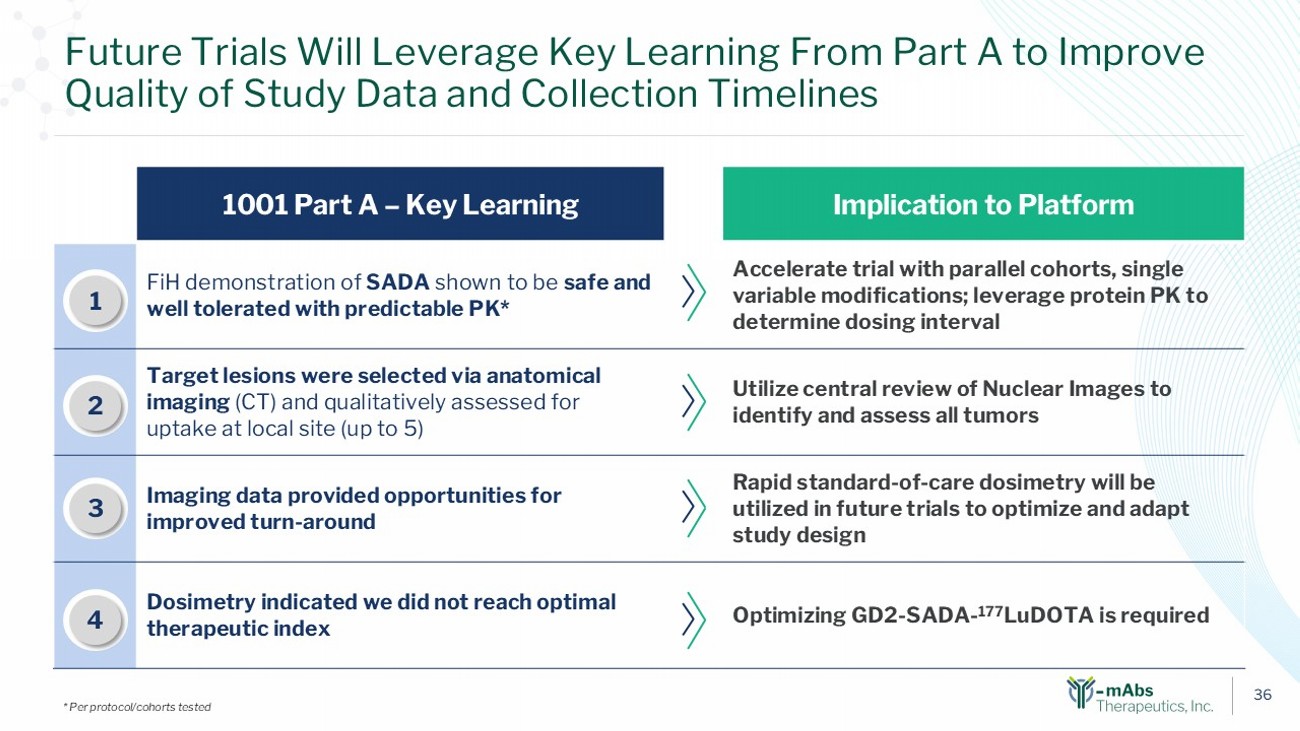
36 Implication to Platform 1001 Part A – Key Learning Accelerate trial with parallel cohorts, single variable modifications; leverage protein PK to determine dosing interval FiH demonstration of SADA shown to be safe and well tolerated with predictable PK* 1 Utilize central review of Nuclear Images to identify and assess all tumors Target lesions were selected via anatomical imaging (CT) and qualitatively assessed for uptake at local site (up to 5) 2 Rapid standard - of - care dosimetry will be utilized in future trials to optimize and adapt study design Imaging data provided opportunities for improved turn - around 3 Optimizing GD2 - SADA - 177 LuDOTA is required Dosimetry indicated we did not reach optimal therapeutic index 1 2 3 Future Trials Will Leverage Key Learning From Part A to Improve Quality of Study Data and Collection Timelines * Per protocol/cohorts tested 4

37 Today’s Agenda: Three Key Radiopharmaceutical Updates 1. Trial 1001 Part A – Complete 2. Key Learnings from Molecule Optimization Studies 3. Expanded Development Pipeline Y - mAbs Development

38 Two Studies Were Conducted in Q1 2025 to Evaluate GD2 - SADA Complex and Identify Opportunities to Improve Tumor Uptake Current Molecule Improved Molecule Y - mAbs 20 pmole (DOTA) Current Molecule Improved Molecule TUMOR KIDNEY Y - mAbs 20 pmole (DOTA) Current Molecule Improved Molecule TUMOR Y - mAbs 20 pmole (DOTA) Current Molecule Improved Molecule KIDNEY Study 1: GD2, 177 Lu, Neuroblastoma Model (2, 24, 48, 96 hr ) 1 Study 2: GD2, Ac225, SCLC Model (2, 24, 48, 96 hr ) 2 1. MSKCC GD2 - SADA Comparison, Q1’25; (Note: improved molecule includes his tag on the GD2 - SADA which was deemed not meaningful t o study results based on testing of other cohort permutations) 2. Minerva Imaging. GD2 SCLC Study with Ac225 Q1’25 Improved Tumor Uptake (~465% vs ~275% ID/g AUC) 1 Improved Tumor Uptake (660% vs 130% ID/g AUC) 2

39 Improved Molecule W ill C onsist of a New Radiohapten and Modified S pecific A ctivity No Change Necessary Change to New Proprietary Radiohapten Change to low Specific Activity (SA) / High Mass No change enables use of existing manufactured protein x Formulation 177 Lu - DOTA Protein Improved tumor uptake over 96 hours Additional studies underway to identify optimal mass levels

40 New Radiohapten Expands Access to a Range of Isotopes with Theranostic Applications, Including Alphas and PET Proprietary R adiohapten creates a “universal linker” to accommodate all payloads with picomolar affinity to anti - DOTA in SADA BsAB (with rapid clearance into the urine) NEW - Proprietary Radiohapten, “PROTEUS” M= • 225 Ac (alpha) • 212 Pb (alpha) • 177 Lu (beta) (SPECT) • 90 Y (beta) • 86 Y (PET) • 89 Zr (PET) • 111 In (SPECT) M Currently In GMP Manufacturing for Clinical Trials

The Improved Molecule Will be Incorporated into a Bridge Study in 1H 2026 * Through a Proposed Amendment ** to the Current IND 1H 2026 – 2H 2026 * • Confirm safety of new Radiohapten in humans • Assess impact of Radiohapten and mass dose on therapeutic index • Optimize clearance intervals (longer retention on tumor) 41 Trial 1001 Bridge Study (Phase 1, Part 2A) 1H 2027 – 2H 2027 * • Identify MTD of Lutetium • Explore OS, PFS, and other efficacy endpoints • Inform patient selection with GD2 - PET imaging Trial 1001 Part B 177 Lu Dose Escalation Trial (Phase 1/2) * Anticipated timing ** New IND vs. Amendment pending FDA feedback; recommendation for new IND may incur delay of 2 - 3 months.

42 Today’s Agenda: Three Key Radiopharmaceutical Updates 1. Trial 1001 Part A – Complete 2. Key Learnings from Molecule Optimization Studies 3. Expanded Development Pipeline Y - mAbs Development

43 We Conducted a Systemic Evaluation to Identify Optimal Targets for the Y - mAbs Platform and Narrowed Selection in 3 Phases 15 Prioritized Targets Staged over Few Years Broad Target Universe Initial refinement – Cancer types of interest (24 cancer types) Second refinement – Tumor Refinement (675 in - scope targets) Assessment with target prioritization framework (45 shortlisted targets) (~1,200) • Incidence Rates • Unmet Need • Radiation Sensitive • Cellular Localization • Tumor Expression • Healthy Tissue Expression • Market Opportunity • Competitive Intensity • Risk - Balanced Platform 1 2 3
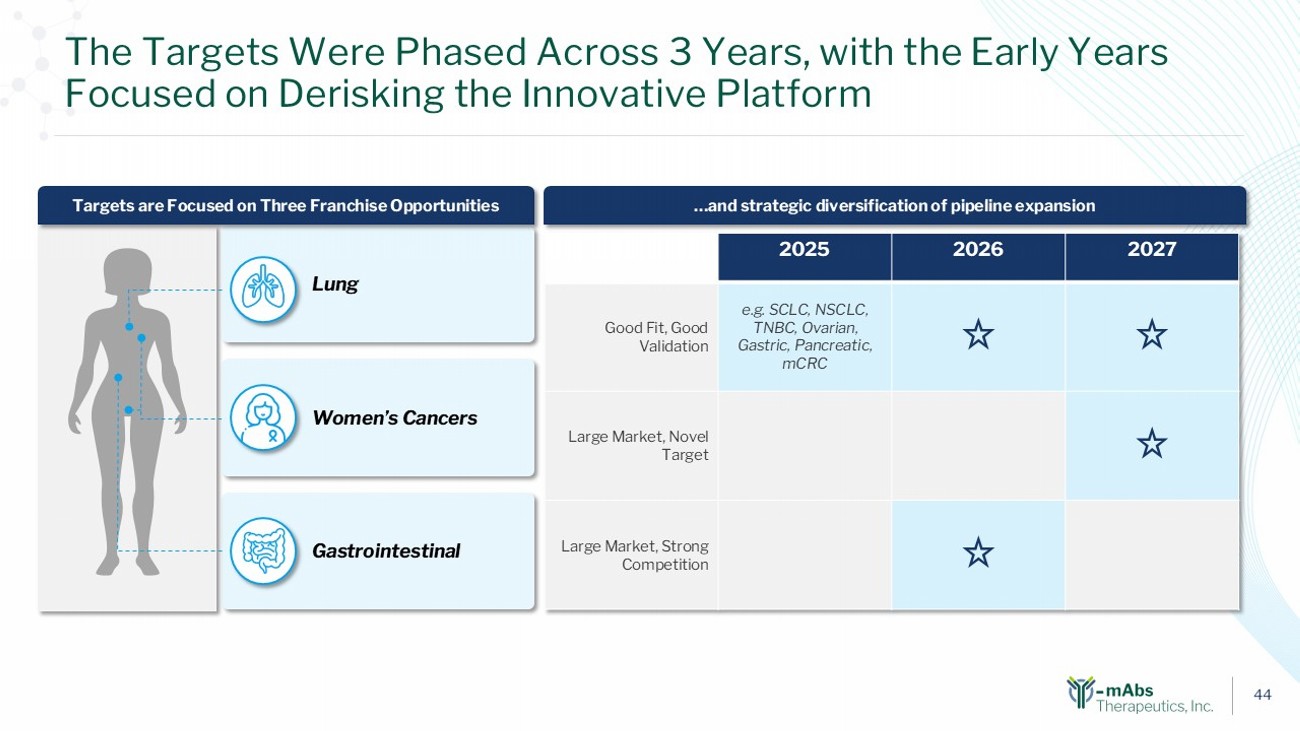
44 The Targets Were Phased Across 3 Years, with the Early Years Focused on Derisking the Innovative Platform …and strategic diversification of pipeline expansion Gastrointestinal Lung Women’s Cancers Targets are Focused on Three Franchise Opportunities 2027 2026 2025 e.g. SCLC, NSCLC, TNBC, Ovarian, Gastric, Pancreatic, mCRC Good Fit, Good Validation Large Market, Novel Target Large Market, Strong Competition

45 Our Radiopharmaceutical Pipeline THERAPEUTIC PIPELINE Phase 2 Phase 1 Preclinical R&D Isotope Asset 177 Lu GD2 - SADA - 177 Lu - Proteus R/R SCLC, Sarcoma, Malignant Melanoma, HR Neuroblastoma GD2 177 Lu CD38 - SADA R/R Non - Hodgkin Lymphoma CD38 Ac225 Antibody Colorectal Cancer Undisclosed Alpha/Beta Antibody Lung, TNBC, Ovarian, Gastro Undisclosed Alpha/Beta Antibody Solid Tumors Undisclosed MOLECULAR IMAGING PIPELINE Phase 2 Phase 1 Preclinical R&D Isotope Asset 89 Zr 89 Zr - DFO - naxitamab R/R SCLC, Sarcoma, Malignant Melanoma, HR NB, Osteosarcoma GD2 89 Zr ( 64 Cu) Undisclosed Colorectal Cancer Undisclosed Undisclosed Undisclosed Lung, TNBC, Ovarian, Gastro Undisclosed Undisclosed Undisclosed Solid Tumors Undisclosed

46 Radiopharmaceuticals: Multiple Potential High Value Inflection Points Anticipated Ahead Increasing commitment, capabilities, and leadership in Radiopharmaceuticals 2025 2026 2027 Realignment into two business units: DANYELZA and Radiopharmaceuticals CD38 - SADA FPI in 1H 2025 GD2 - SADA Trial 1001 Part A Data Readout GD2 - Diagnostic IND Submission in 2H 2025 GD2 - Diagnsotic FPI 1H 2026 GD2 - SADA 1001 IND Amendment* 1H 2026 Initiate GD2 - SADA Bridge Study with new Radiohapten in 1H 2026 Trial 1001 Bridge Study Data Readout with new Radiohapten in 2H 2026 Initiate Dose Escalation Study (Trial 1001, Part B) in 1H 2027 Initiation of GD2 - SADA Pediatric Study (Trial 1002) in 1H 2027 GD2 - SADA Pediatric Trial (Trial 1002) Data Readout in 2H 2027 GD2 - SADA Dose Escalation Study (Trial 1001, Part B) Data Readout in 2H 2027 NEW TARGET: IND submission (mCRC) in 1H 2027 NEW TARGET: FPI New Therapeutic (mCRC) 2H 2027 2024 Increased organizational focus on Radiopharmaceuticals New Executive Team appointed with deep Radiopharma expertise * New IND vs Amendment pending FDA feedback; recommendation for new IND may incur delay of 2 - 3 months

47 In Conclusion: Recent Insights Will Be Scaled Across the Platform GD2 - SADA Protein is safe and well - tolerated Protein PK and dosing interval optimize the Therapeutic Index New Universal Radiohapten expected to modularize the platfor m, allow for multiple isotopes, and improve tumor retention New targets expand value opportunity by addressing large unmet medical needs Safe platform, predictable PK and improved operations will accelerate development

Q&A 48

Thank You 49
Exhibit 99.2

Y-mAbs
Hosts Virtual Radiopharmaceutical R&D Update
Highlighting Clinical Progress and Expanded Pipeline
- Company’s Part A data readout from first-in-human Phase 1 Trial 1001 in patients with recurrent or refractory metastatic solid tumors known to express GD2, validates GD2-SADA as safe, tolerable and able to achieve targeted in vivo conjugation of 177Lu-DOTA
- Increased tumor retention and total tumor uptake anticipated by using optimized universal Radiohapten
- Company plans to initiate a Trial 1001 Bridge study (Part 2A) with optimized Radiohapten, “Proteus”, in 1H 2026 with data readout in 2H 2026; Part B of Trial 1001 anticipated to initiate with Proteus in 1H 2027 with data readout in 2H 2027
| - | Expanded Radiopharmaceutical pipeline to focus on target franchise areas in oncology, with specific programs that maximize pretargeting approach in high-value commercial targets |
| - | Company to host virtual Radiopharmaceutical R&D update today at 8:00 a.m. ET |
Princeton, NJ, May 28, 2025 – Y-mAbs Therapeutics, Inc. (the “Company” or “Y-mAbs”) (Nasdaq: YMAB), a commercial-stage biopharmaceutical company focused on the development and commercialization of novel radiopharmaceuticals, and commercial stage antibody-based therapeutic products for the treatment of cancer, today announced that the Company plans to highlight progress across its Radiopharmaceutical Business Unit during a virtual R&D update to be held today, Wednesday, May 28, 2025 at 8:00 a.m. ET.
“At Y-mAbs, our mission is to deliver innovative therapeutic solutions for life-threatening diseases and improve the lives of patients and their families,” said Michael Rossi, President and Chief Executive Officer. “We are excited to provide these updates across our Radiopharmaceutical Business today, share data confirming our pretargeted approach has been validated in humans, and reiterate the potential of our platform to deliver novel products that we believe will have a meaningful impact on how we treat certain cancers. Based on today’s update, we reaffirm our commitment to accelerating the clinical advancement of our Self-Assembly DisAssembly Pretargeted radioimmunotherapy (“SADA PRIT”) technology platform and pipeline.”
“The complete Part A data from Trial 1001 highlighted today provides further validation for our novel SADA PRIT technology platform,” said Natalie Tucker, Radiopharmaceutical Business Unit Head. “This data from Part A of Trial 1001 adds to the substantial learning we have developed through clinical and preclinical research regarding our SADA PRIT technology. Based on our work, we believe that SADA is a truly differentiated pretargeted platform positioned to potentially disrupt the radiopharmaceutical industry and significantly improve patient outcomes.”
Radiopharmaceutical R&D Update Highlights
GD2-SADA Phase 1 Clinical Trial (Trial 1001): Part A Completed
| · | The primary objective of Trial 1001 is to evaluate the safety and tolerability of GD2-SADA in adult and adolescent patients with recurrent or refractory metastatic solid tumors, including small cell lung cancer, sarcomas, malignant melanomas, and high-risk neuroblastoma. In Part A, the Company first explored variable protein doses of 0.3, 1.0, and 3.0 mg/kg and a pre-targeting interval of two to five days. |
| · | Of the 22 patients dosed with both the GD2-SADA Protein and 177Lu-DOTA, nine patients had positive GD2 expression, per protocol, and were eligible for the therapeutic stage of the study to receive up to 200 mCi of 177Lu-DOTA. |

| · | The initial concentration of administered GD2-SADA Protein correlated with the amount of GD2-SADA in serum at the Cmax and over time (AUC). |
| · | The GD2-SADA Protein PK was highly reproducible within cohorts, and when normalized by dose concentration similar Cmax and clearance rates were observed over time. |
| · | These results demonstrate that the GD2-SADA Protein clearance rate is reliably correlated to dose concentrations and PK provides a roadmap for tailoring the clearance interval prior to isotope administration. |
| · | Higher concentrations of 177Lu-DOTA were correlated with higher GD2-SADA Protein concentrations, indicating effective targeting of the 177Lu-DOTA to GD2-SADA. |
| · | Part A of Trial 1001 demonstrated positive tumor uptake and quantifiable absorbed dose to the tumor at 30 mCi. |
| · | Both the GD2-SADA and 177Lu-DOTA administrations were generally safe and well-tolerated. No treatment-related serious adverse events occurred across all dosing cohorts and there were no reports of serious treatment-related pain that has been historically associated with dosing of anti-GD2 therapies. |
SADA Optimization Data
| · | The Company completed a number of pre-clinical studies over the last few quarters to evaluate multiple GD2-SADA-177Lu-DOTA molecule constructs for optimizing tumor-to-organ ratios. Results from this extensive work have demonstrated that the 177Lu-DOTA and molecule formulation can be optimized to improve tumor uptake and retention. Accordingly, the Company has chosen to move forward with “Proteus,” a novel universal radiohapten which has demonstrated the potential to expand access to a range of isotopes with theranostic applications. |
| · | Y-mAbs is committed to advancing its GD2-SADA program and achieving accelerated validation of Proteus to leverage across its platform and new target programs. The Company plans to file an amendment to its current IND for Trial 1001 to incorporate Proteus for a Bridge study (Part 2A) as part of Trial 1001. The Bridge study aims to assess the safety of Proteus in patients and the impact of mass dose on the therapeutic index. The Company anticipates initiating the Bridge study in the first half of 2026. |
| · | Following completion of the Bridge study, Y-mAbs anticipates launching the dose escalation portion of Trial 1001, Part B, which is expected to be a Phase 1/2 clinical trial, in the first half of 2027 with data in the second half of 2027. |
Expanded Radiopharmaceutical Development Pipeline
| · | Following a systematic evaluation to identify optimal targets for its novel SADA platform, Y-mAbs has selected lung cancer, women’s cancers, and gastrointestinal cancers as target oncology franchise-expanding opportunities. In addition, the Company has established a discovery and pre-IND molecular imaging portfolio, complementary to its planned therapeutic portfolio. The Company anticipates filing an IND for its first molecular imaging asset by the end of 2025. |
Webcast Information
The duration of the virtual Radiopharmaceutical R&D update is expected to be 90 minutes. A live audio webcast of the call will be available on the Investor Relations section of the Company’s website at https://ir.ymabs.com/events-and-presentations/events. The webcast will be archived for at least 30 days.

About Y-mAbs
Y-mAbs is a commercial-stage biopharmaceutical company focused on the development and commercialization of novel, radioimmunotherapy and antibody-based therapeutic cancer products. The Company’s technologies include its investigational Self-Assembly DisAssembly (“SADA”) Pretargeted Radioimmunotherapy Platform (“PRIT”) and bispecific antibodies generated using the Y-BiClone platform. The Company’s broad and advanced product pipeline includes the anti-GD2 therapy DANYELZA® (naxitamab-gqgk), the first FDA-approved treatment for patients with relapsed or refractory high-risk neuroblastoma in the bone or bone marrow after a partial response, minor response, or stable disease to prior therapy.
About GD2-SADA PRIT
GD2-SADA is a bispecific fusion protein that tightly binds to the glycolipid GD2 and Lutetium 177 (Lu 177)-DOTA, a chelated or “caged” radionuclide. In the first step of pre-targeted radiotherapy, non-radiolabeled GD2-SADA tetramers are infused and bind to GD2-expressing solid tumors, while unbound GD2-SADA protein disassembles into low molecular weight monomers that are removed by the kidney. The second infusion delivers the “radioactive payload,” which binds directly to GD2-SADA on tumor cells for localized irradiation. GD2-SADA PRIT with 177Lutetium-DOTA has demonstrated anti-tumor activity in preclinical studies and is currently being investigated in adults and adolescents with GD2-expressing solid tumors in Trial 1001 (NCT05130255).
Researchers at Memorial Sloan Kettering Cancer Center (MSK), including Dr. Nai-Kong Cheung, developed the SADA technology for radioimmunotherapy, which is exclusively licensed by MSK to Y-mAbs. Dr. Cheung has intellectual property rights and interests in the technology, and as a result of this licensing arrangement, MSK has institutional financial interests in the technology.
Forward-Looking Statements
Statements in this press release about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements include, but are not limited to, statements about the clinical development of the Company’s Radiopharmaceutical product candidates, including the progress of and results from ongoing clinical trials and the timing of initiation of additional clinical trials; the potential of the Company’s SADA technology to disrupt the radiopharmaceutical industry and significantly improve patient outcomes; and the timing of regulatory filings for the Company’s product candidates and expectations regarding the expansion of its oncology franchise. Words such as ‘‘anticipate,’’ ‘‘believe,’’ “contemplate,” ‘‘continue,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ “hope,” ‘‘intend,’’ ‘‘may,’’ ‘‘might,’’ ‘‘plan,’’ ‘‘potential,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘should,’’ ‘‘target,’’ “will,” ‘‘would’,’ “guidance,” “goal,” “objective,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Our product candidates and related technologies are novel approaches to cancer treatment that present significant challenges. Actual results may differ materially from those indicated by such forward-looking statements as a result of various factors, including but not limited to: risks associated with the Company’s financial condition and need for additional capital; the risks that actual results of the Company’s recent business realignment will not be as expected; risks associated with the Company’s development work, including any delays or changes to the timing, cost and success of our product development activities and clinical trials including if we encounter difficulties enrolling patients in our clinical trials; the risks of delay in the timing of the Company’s or its partners’ regulatory submissions or failure to receive approval of its drug candidates; the risks related to commercializing any approved pharmaceutical product including the rate and degree of market acceptance of product candidates; development of sales and marketing capabilities and risks associated with failure to obtain sufficient reimbursement for products; risks related to the Company’s dependence on third parties including for conduct of clinical testing and product manufacture as well as regulatory submissions; the Company’s ability to enter into new partnerships or to recognize the anticipated benefits from its existing partnerships; risks related to government regulation; risks associated with protection of the Company’s intellectual property rights; risks related to employee matters and managing growth; risks associated with macroeconomic conditions, including the conflict between Russia and Ukraine and Israel and Hamas and sanctions related thereto, international trade policies, including tariffs and trade restrictions, inflation, increased interest rates, uncertain global credit and capital markets and disruptions in banking systems; and other risks and uncertainties affecting the Company including those described in the “Risk Factors” section included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024, the Company’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2025, and future filings and reports by the Company. Any forward-looking statements contained in this press release speak only as of the date hereof, and the Company undertakes no obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.

SADA®, SADA PRIT™, DANYELZA® and Y-mAbs® are registered trademarks of Y-mAbs Therapeutics, Inc.
Investor Contact:
Courtney Dugan
VP, Head of Investor Relations
